 |
 |

Incidence of Adverse Drug Reactions in Hospitalized Patients
A Meta-analysis of Prospective Studies
Jason
Lazarou, MSc; Bruce H. Pomeranz, MD, PhD;
Paul N. Corey, PhD
JAMA. 1998;279:1200-1205.
ABSTRACT
 |
 |
Objective. To estimate the incidence of serious and fatal
adverse drug reactions (ADR) in hospital patients.
Data Sources. Four electronic databases were
searched from 1966 to 1996.
Study Selection. Of 153, we selected 39 prospective
studies from US hospitals.
Data Extraction. Data extracted independently by 2 investigators were analyzed
by a random-effects model. To obtain the overall
incidence of ADRs in hospitalized patients, we combined the incidence of ADRs occurring while in the hospital plus the incidence of ADRs
causing admission to hospital. We excluded
errors in drug administration, noncompliance, overdose,
drug abuse, therapeutic
failures, and possible ADRs. Serious ADRs were
defined as those that required
hospitalization, were permanently disabling, or resulted
in death.
Data Synthesis. The overall incidence of serious ADRs
was 6.7% (95% confidence interval [CI], 5.2%-8.2%) and of
fatal ADRs was 0.32% (95% CI, 0.23%-0.41%) of
hospitalized patients. We estimated that
in 1994 overall 2216000
(1721000-2711000) hospitalized patients had serious ADRs and 106000
(76000-137000) had fatal ADRs, making these reactions between the fourth and
sixth leading cause of death.
Conclusions. The incidence of serious and fatal
ADRs in US hospitals was found to be
extremely high. While our results must be viewed with
circumspection because of heterogeneity among studies and
small biases in the samples, these data
nevertheless suggest that ADRs represent an important
clinical issue.
INTRODUCTION
PUBLIC
ATTENTION is currently focused on adverse drug reactions (ADR) as
evidenced by a recent bill passed by the US Senate
requiring pharmaceutical companies to
provide ADR information to
consumers.1
Heightened interest in ADRs was stimulated by
the thalidomide tragedy in the 1960s.2
To obtain an accurate estimate of
ADR incidence in hospital patients, prospective
studies were done, beginning in the 1960s, in which a defined population could be
kept under close observation by monitors who recorded all
ADR occurrences.3-5
These prospective studies have been done on 2 separate
populations of patients; those admitted to
the hospital due to an ADR (ADRAd),6
and those experiencing an ADR while
in the hospital (ADRIn).7
We report here a meta-analysis of 39 of these prospective
studies done in the United States over a
period of 32 years from which we obtained ADR incidences for
ADRIn and for ADRAd and an overall ADR
incidence that combines these 2 groups. We
focused mainly on serious and fatal ADRs
since they represent the greatest
impact of drug therapy. While
recognizing the benefits of drug therapy, we chose not to
compare benefits of drugs to the side effects of
drugs.
METHODS
Definitions
One step we took to reduce heterogeneity was to exclude any
data that did not use the following specific definitions:
Adverse Drug Reaction (ADR)
According to the World Health Organization
definition,8
this is any noxious, unintended, and undesired effect of a
drug, which occurs at doses
used in humans for prophylaxis,
diagnosis, or therapy. This definition excludes therapeutic failures,
intentional and accidental
poisoning (ie, overdose), and drug abuse.8
Also, this does not include adverse events due to errors
in drug administration or
noncompliance (taking more or less of a drug than the prescribed
amount).8
Using this conservative definition avoids
overestimating the ADR incidence.
Recently, some authors prefer the term adverse drug event (ADE), which
is an injury resulting from administration of a drug. In contrast to the World Health
Organization definition of ADR, the
definition of ADE includes errors in administration.9
However, we have chosen the World Health Organization
definition for ADR because of
its frequent use in the studies that we analyzed,
and because of our goal to estimate injuries incurred by drugs that were properly
prescribed and administered. In those articles that did
not use the World Health Organization definition (eg, ADE was used),
we examined the raw data and removed
adverse events due to
errors in administration. However, this was
not always feasible since a few articles may have
included errors
in administration but did not report them
separately. Therefore, unfortunately, these latter
articles added to the heterogeneity of our data.
Possible ADR
This is an ADR that follows a reasonable temporal sequence
and for which the ADR is a known response to the
drug, although the response
may also be explained by the patient's clinical state.10
Possible ADRs were excluded from our study.
Serious ADR
This is an ADR that requires hospitalization, prolongs
hospitalization, is permanently disabling, or results in death. Serious ADRs
include fatal ADRs, which were also
analyzed separately.
Prospective Studies
Patients were present during the study, and monitors were
able to interview physicians, nurses, or
patients at least once per
week. All ADRs were confirmed prior to patient's discharge
from the hospital.
Retrospective Studies
Chart reviews were performed after the patient had left the
hospital. These studies were excluded from our analysis.
Literature Search
Electronic databases were searched using the following key word strategy:
adverse drug or adverse reaction or
drug-related or
drug-induced and hospital. Three
MeSH (Medical Subject Headings) terms were also used
where appropriate (ie, hospitalization, drugs, drug therapy/adverse effects) in combination with key words.
Databases that we used were MEDLINE (1966-1996), Excerpta
Medica (1980-1996), International Pharmaceutical Abstracts
(1970-1996), and Science Citation Index (1989-1996). The reference
sections of all retrieved articles were manually searched
for additional studies. In addition, we sent letters to
researchers in the field to request
unpublished data in order to reduce publication
bias.
Selection Criteria
The following criteria were used:
- The patients studied were not selected
for particular conditions or specific drug exposures.
- Sufficient information was reported
in the published study to
calculate the incidence of ADRs.
- English translations of the papers were
available.
- Prospective monitoring was used to identify
ADRs.
- Definitions used in the studies
coincided with ours (see
"Definitions" subsection for
our definitions).
Quality of the Data
Rather than merely assessing the quality of each study,11
we chose instead to improve the quality of our
database. First, we used prospective monitoring as an inclusion criterion to
exclude the lowest-quality studies (ie, the retrospective
studies). Second, ADRs classified as "possible" were
excluded. Attributing causality is always a
problem with ADR detection12
and, by excluding possible ADRs, we
reduced the number of false positives in the data.
Heterogeneity
We dealt with heterogeneity among the studies in numerous ways: (1) we
placed considerable emphasis on the 95% confidence intervals (CIs) to draw
attention to the heterogeneity,13
(2) we used a random-effects model to do the analysis
because it takes into account the
heterogeneity of the various studies,13-14
(3) to reduce heterogeneity, we excluded ADRs caused by
errors in administration, noncompliance,
overdose, drug abuse, or therapeutic
failures, (4) for additional ways to reduce
heterogeneity, we excluded ADRs not fitting our strict definitions, possible ADRs, and
retrospective data.
Data Extraction
We determined the incidence of ADRs in the hospital by
extracting the total number of
hospital patients in each study experiencing at least 1 ADR and
dividing this value by the total number
of hospital patients in each study. The ADR incidence was expressed as
the percent of patients with an ADR. A data
collection form was developed prior to the study for this
purpose. Information on nonserious,
serious, and fatal reactions was extracted. Other
data extracted included the year of the study, ward
and hospital type in which the study was performed, mean
age, average length of hospital stay, average number of
drug exposures for the patients included in the study, and the number of men
and women in each study. To test for
reliability of our extraction procedures a randomly
selected subset of the data was extracted independently by 2 of us
(J.L. and B.H.P.) and was found to be very consistent for
the published ADR incidence for serious, fatal, and
all severities (intraclass correlation coefficient
ranging from 0.89 to
0.92).
Analysis of ADR Incidence
We separately analyzed the incidence of ADRIn and the incidence of ADRAd and then
combined the 2 groups to obtain an overall ADR
incidence. We analyzed ADRs of all
severities (which included nonserious and
serious), ADRs that were serious (which included fatal), and ADRs
that were fatal; however, we focused mainly on the serious and
fatal ADRs. For each category, we analyzed the ADR
incidences obtained from the different studies to
determine the mean incidence and the 95% CIs. For this
purpose we used a random-effects model for
meta-analysis15
similar to the method used in the only previous meta-analysis of
ADRAds.16
This is the method of choice because it takes
into account the heterogeneity
of the various studies.14
When combining the incidence of ADRIn and ADRAd to obtain the overall
incidence of ADRs, we avoided double
counting patients who were admitted
for an ADR and who then also experienced an ADR while
in the hospital by assuming the 2 types of events
to be independent and deriving an adjusted estimate
using the following formula:
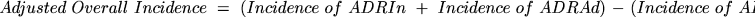
This provided a slightly smaller estimate of the ADR
incidence. For example, the
mean estimate for the overall number of serious ADRs per
year (see "Results" section) would change by 33000
patients, dropping from 2249000 (no adjustment) to
2216000 (our estimate using the adjustment).
When comparing groups, we used both parametric
and nonparametric methods. The results were always the
same for the 2 methods. Hence, for group comparisons,
whenever possible, we reported the results of the more
robust nonparametric Wilcoxon rank sum test.17
All statistical analyses were performed using the SAS statistical
software package, version 6.11 (Statistical Analysis
System, Cary, NC).
Number of Patients With ADRs
We estimated the number of hospital patients with ADRs in the United States by
using the incidence of ADRs in US hospitals derived
from our data and multiplying this value by the number
of hospital admissions in 1994 in the United States,
obtained from published
statistics.18
In 1994, there were 33125492
hospital admissions in the United States. We calculated
the 1994 fatal ADRIns as follows:
Number of Fatal ADRIns in US Hospitals in 1994 (63000)=Incidence of Fatal
ADRIns in Hospitals in the United States (0.0019)xNumber of Hospital
Admissions in the United States (33125492).
This estimate is based on the assumption that our sample is
representative of the hospital population, and, hence, we
examined representativeness at
some length (see "Results" section).
RESULTS
Using our 5 selection criteria, 39 of
the 153 studies found in the literature were included in our meta-analysis. Features
of these 39 studies are given in Table
1 and Table
2.4-7,9,
19-43
Fifty-seven studies were excluded from our meta-analysis
by the 2 blinded investigators because they did not
meet our criteria. In addition 57 of the
remaining 96 studies were performed
in countries other than the United
States and were excluded from our meta-analysis because
one of our major goals was to determine representativeness of our sample
in order to establish the
accuracy of our summary statistics. Since we only had a
sufficient number of studies from the United States to
allow us to perform these tasks, we decided to exclude
the remaining countries from our
meta-analysis since a proper analysis for
representativeness for any other country would be
impossible to perform.
|
|
 |
| Table 1.Studies on ADRs in Patients While
in the Hospital
(ADRIn)*
| | |
|
|
 |
Table 2.Studies on Patients Admitted to
the Hospital Due to an ADR*
| | |
Incidence of ADRs
As shown in Table
3, the incidence of serious ADRIn was 2.1% (95% CI,
1.9%-2.3%) of hospital patients, while the incidence of serious ADRAd
was 4.7% (95% CI, 3.1%-6.2%). The incidence of fatal
ADRIn was 0.19% (95% CI, 0.13%-0.26%) of
hospital patients and the
incidence of fatal ADRAds was 0.13%
(95% CI, 0.04%-0.21%). Combining ADRIn and ADRAd, the overall
incidence of serious ADR
was 6.7% (95% CI, 5.2%-8.2%) of hospital patients and the overall
incidence of fatal ADRs was 0.32% (95%
CI, 0.23%-0.41%). The incidence of ADRIn of all severities (including nonserious and serious)
was 10.9% (95% CI, 7.9%-13.9%) of hospital patients. The overall
incidence of ADRIn plus ADRAd for ADRs of all
severities was 15.1% (95% CI, 12.0%-18.1%) of hospital
patients.
|
|
 |
| Table 3.ADR Incidence
According to ADR Severity*
| | |
Eight ADRIn articles included the proportion of type
A44
(dose-dependent ADRs) and type B44
(idiosyncratic and/or allergic ADRs). Of the "all
severities" ADRIn, 76.2% (95% CI, 71.0%-81.4%)
were type A reactions and 23.8% (95% CI,
18.6%-29.0%) were type B reactions. Unfortunately, none of
these studies reported the proportion of type A and type
B reactions for serious and fatal
ADRs.
Number of Hospital Patients With ADRs
As shown in Table
4, we estimated that 702000 (95% CI, 635000-770000)
hospital patients in the United States experienced a
serious ADRIn in 1994. We calculated that 1547000
(95% CI, 1033000-2060000) hospital patients experienced a serious ADRAd.
Combining these values, overall
2216000 (95% CI, 1721000-2711000) hospital patients experienced a
serious ADR in the United States in 1994. We calculated that
there were 63000 (95% CI, 41000-85000) fatalities due to
ADRIn and another 43000 (95% CI,
15000-71000) deaths occurred in association with ADRAd
in the United States. Overall
in 1994, we estimated that
106000 (95% CI, 76000-137000) deaths were caused by ADRs
in the United States, which could
account for 4.6% (95% CI, 3.3%-6.0%) of the 2286000
recorded deaths from all causes during 1994 in the United States.18
Using the mean ADR
incidence (106000) or the more
conservative lower 95% CI (76000), we found that fatal
ADRs ranked between the fourth and sixth
leading cause of death in the United States in 1994.
|
|
 |
Table 4.Estimated Number of Hospital
Patients
in 1994 With ADRs,
in Thousands (95%
CI)*
| | |
Representativeness of Our Sample
Among the many factors possibly influencing ADR incidence, considerable
research has identified average length of stay,45-46
age,45,
47
gender,48-49
and drug exposure.45-46
Therefore, as shown in Table
5, we checked to see whether the population that we
sampled was representative of the US hospital
population50
vis-ΰ-vis these 4 factors. We determined that the differences were
significant for length of stay and gender but not for
age. Unfortunately, we were unable to find values for the average number of
drug exposures from
national statistics. Possible biases in our ADR incidence that may have been caused by
the differences in length of stay or gender
are estimated in the "Comment" section.
|
|
 |
| Table 5.Is Our Sample Representative of US
Hospitals?
| | |
Another possible source of sampling bias might be the year of
study, as our meta-analysis spans 4 decades. Hence, we
studied the relationship between ADR incidence and year of study
using a random-effects
linear regression model and found no
significant correlation for ADRIn (r=0.27, P=.14, n=18)
or for ADRAd (r=0.23, P=.34, n=21). Figure
1 shows these results graphically and indicates that no change
in ADR incidence occurred over the span of
our study. This result seems surprising since great changes have
occurred over the last 4 decades in US hospitals that should
have affected the incidence of ADRs. Perhaps, while
length of hospital stay is decreasing,51
the number of drugs per day may be
rising to compensate. Therefore, while
the actual incidence of ADRs has not
changed over the last 32 years, the pattern of their
occurrence has, undoubtedly, changed.
|
|
 |
| Incidence of
adverse
drug reactions (ADRs)
in 39 studies
distributed over 32 years. All 39 points are not visible
as several are superimposed on each other.
Linear regression,
using a random-effects
model, showed no significant correlation for
either those experiencing an ADR while
in the hospital
(ADRIn) (r=0.27,
P=.14) or those admitted to the hospital
due to an ADR (ADRAd) (r=0.23,
P=.34).
| | |
It should be noted that additional factors have been
proposed to have an effect on ADR rate: renal function,
hepatic function, alcoholism, drug abuse, and severity of
illness.44,
52
Unfortunately, these factors were rarely reported
in our sample of studies
and, thus, could not be used to determine representativeness.
Medical wards are overrepresented in our database, and some
articles in the literature suggest that ward
type might have an effect on ADR incidence.9,
40,
53-54
Unfortunately, there is insufficient power in the 39 studies to calculate the
incidence of ADRs for each
ward type individually. Without these data,
we cannot determine the possible effect that ward-type
distribution might have on our ADR incidence. Nevertheless, in the "Comment" section,
we estimate the possible bias due to ward type.
Similar to ward type, hospital type may also introduce bias
into our results. It is thought that
teaching hospitals contain more seriously ill
patients than nonteaching hospitals, which may
lead to a higher incidence of ADRs in teaching hospitals, but this has
never been proven.35,
55
Teaching hospitals are
overrepresented in our sample. However, when we
compared ADR incidences for teaching and nonteaching hospitals in our study, we found no
significant differences. Thus, despite an overrepresentation
of teaching hospitals in our sample, there may not be a
major bias.
Finally, our letters to researchers
in the field produced no
evidence of publication bias.
COMMENT
We have
found that serious ADRs are frequent and more so than
generally recognized. Fatal ADRs appear to be between the
fourth and sixth leading cause of death. Their
incidence has remained stable over the last
30 years.
There has been only one previous meta-analysis of ADR
hospital studies,16
and it focused only on ADRAd. Our article differs from
this report in many respects: (1) we studied
incidence of
ADRIn as well as ADRAd, (2) we
combined ADRAd and ADRIn to obtain the overall incidence of ADRs, (3) we gave special
emphasis to serious and fatal ADRs, (4) we improved the
quality of the data by excluding retrospective studies and by
excluding ADRs that were
classified as "possible," (5) we examined the representativeness
of our sample, and (6) we estimated the total number of
patients in US hospitals experiencing ADRs.
Recent studies have focused on ADEs, which include errors in administration.9,
19-20
One of the goals of ADE research is to alert physicians
about the preventability of many ADEs.20
In contrast, our study on ADRs, which
excludes medication errors, had a different objective: to
show that there are a large number of serious ADRs even
when the drugs are properly prescribed
and administered.
We found that a high proportion of ADRs (76.2%) were type A
reactions. This may suggest that many
ADRs are due to the use of drugs with unavoidably high toxicity.
For example, warfarin often results
in bleeding. It has been shown that careful
drug monitoring in hospitals leads to a reduction of
many of these ADRs, suggesting that some type A and type B ADRs
may be due to inadequate monitoring of therapies and doses.56
Recent studies have shown that the costs associated with
ADRs may be very high. Research to determine the hospital costs directly
attributable to an ADR estimated that ADRs may lead to an
additional $1.56 to $4 billion in direct hospital costs per year
in the United
States.57-58
Heterogeneity
As outlined in the "Methods" section, we dealt
with heterogeneity in numerous ways. After
taking these measures, we
examined the remaining heterogeneity. We
determined whether 4 factors thought
to affect ADR incidence (age, gender, drug exposure, and length
of stay) contributed to the remaining heterogeneity in our data using a linear regression version of the
random-effects model.15
For ADRIn, we found that number of
drug exposures and length
of hospital stay jointly accounted for 43% of the
variance (r=0.65, P=.009, n=18). For the
rate of ADRAd, when age was included in the model, the variance was reduced
by 27% (r=0.52, P=.04, n=14). Gender did
not contribute to the variance. Thus, a great deal of the
heterogeneity could be attributed to factors well known
to affect ADR rates: number of drug exposures per patient,
length of hospital stay, and the age of patients. This result
indicates that much of the
heterogeneity is due to variation in the populations examined in the various articles and,
hence, only a portion of the variation could merely be
attributed to inconsistent methods among the
individual studies. For example,
if the different investigators use different methods of
ascertainment regarding what represents an ADR, they will
find different rates.
Another example of inconsistent methodology is the
problem that some articles did not separate out
administration errors.
Methodological variation such as this is a limitation of
meta-analysis.
Representativeness of Our Sample
In the "Results" section, we found
that for the 5 factors examined 3 were possible
sources of bias: length of stay, gender, and ward type.
Thus, we have attempted to estimate the size of the
sampling bias due to these 3 factors as
follows. As seen in Table
5, we had a higher average length of hospital stay than
the US national average (10.6 days vs 7.6 days).18
While the literature qualitatively reports a relationship
between the incidence of ADRIn and length of stay,21,
45-46
there are no quantitative estimates. Therefore, we
performed a linear regression analysis
on our own data using a random-effects model15
regressing the incidence of ADRIn of all severities on average length
of stay to obtain a slope of 0.007 (P=.008)
and deduced that increasing the length of hospital
stay from 7.6 to 10.6 days would possibly cause the
incidence of ADRIn of all severities to rise from
the adjusted value of 8.7% to our value of 10.9%.
Also, as shown in Table
5, the proportion of female patients in our sample was lower than the
national average (50% vs 60%). Using several studies
reporting an increased incidence of ADRs among
females, we were able to determine that, at most, the risk
ratio for women vs men could be as high as 1.5 for both
ADRIn and ADRAd. Assuming the worst-case scenario, the
adjusted value for the overall incidence of ADRs of all severities
in the United States
becomes 15.7% (95% CI, 12.7%-18.8%) compared with our
value of 15.1% (95% CI, 12.0%-18.1%).
Finally, with regard to ward type,
there was insufficient power
in 39 studies to determine precisely the effect of
ward-type discrepancies. Instead, we made a crude
determination of the worst-case
scenario of ward bias. If we assumed (1) that obstetrical
wards have zero ADRs and (2) that we sampled zero
obstetrical patients, and, since there are about 4 million
obstetrical ward patients each year in the United States59
of 33 million total hospital admissions,18
then the total number of ADRs occurring in the United States would be 4/33
lower than our estimates. Thus the overall number of
fatal ADRs in the United States would
drop from 106000 (95% CI, 76000-137000) to 93000 (95% CI,
67000-121000), which would make ADRs between the fourth
and seventh leading cause of death
in the United States rather than
between the fourth and sixth leading cause as reported above.
Regarding other ward types,
psychiatric wards tend to have a higher ADR incidence and pediatric wards a lower
ADR incidence than medical
wards,53-54
so these 2 biases might cancel out. Thus, altogether,
there probably is a small net upward bias in our ADR incidence due to our
overrepresentation of medical wards.
It is important to note that we have taken a conservative
approach, and this keeps the ADR estimates low by
excluding errors in administration, overdose, drug abuse, therapeutic failures,
and possible ADRs. Hence, we are probably not
overestimating the incidence of ADRs despite the 3 small
sampling biases discussed
earlier.
CONCLUSIONS
Perhaps, our
most surprising result was the large number
of fatal ADRs. We estimated that in 1994 in the United States 106000
(95% CI, 76000-137000) hospital patients died from an ADR. Thus,
we deduced that ADRs may rank from the fourth to sixth
leading cause of death. Even if
the lower confidence limit of 76000 fatalities was used
to be conservative, we estimated that ADRs could still
constitute the sixth leading cause of death in the United States, after
heart disease (743460), cancer (529904), stroke (150108),
pulmonary disease (101077), and accidents (90523); this
would rank ADRs ahead of pneumonia (75719) and diabetes
(53894).18
Moreover, when we used the mean value of 106000 fatalities,
we estimated that ADRs could rank fourth, after heart
disease, cancer, and stroke as a leading cause of death. While our
results must be viewed with some circumspection because
of the heterogeneity among the studies and small biases
in the sample, these data
suggest that ADRs represent an important clinical issue.
AUTHOR INFORMATION
This work
was supported by a grant (Dr Pomeranz) and a scholarship
(Mr Lazarou) from the National Science Engineering Research Council,
Ottawa, Ontario.
J. L. Lazarou did this work in partial fulfillment of his MSc
degree at the University of Toronto, Ontario; B. H.
Pomeranz, MD, PhD, was the principal investigator; and P. N. Corey,
PhD, was the statistician who contributed to the
conception, design, analysis and interpretation of the data, and also
particpated in writing the manuscript.
A complete list of the 104 papers excluded from our
meta-analysis is available on request from the
authors.
Reprints: Bruce H. Pomeranz, MD, PhD,
Departments of Physiology and Zoology, University of
Toronto, 25 Harbord St, Toronto, Ontario, Canada M5S 3G5
(e-mail: pomeranz{at}zoo.utoronto.ca
).
From the Departments of Zoology (Mr Lazarou and Dr Pomeranz),
Physiology (Dr Pomeranz), and Public Health Sciences (Dr Corey),
University of Toronto, Toronto, Ontario.
REFERENCES
 |
 |
1.
Gray J. Bill would force drug makers to give customers data on
risks. New York Times. July 25, 1996:A11.
2.
D'Arcy PF, Griffin JP. Thalidomide revisited.
Adverse Drug React Toxicol Rev.
1994;13:65-76. ISI |
PUBMED
3.
Cluff LE, Thornton CF, Seidl LG. Studies on the epidemiology of
adverse drug reactions, part 1: methods of
surveillance. JAMA. 1964;188:976-983. ISI |
PUBMED
4.
Seidl LG, Thornton GF, Smith JW, Cluff LE. Studies on the
epidemiology of adverse drug reactions, III: reactions in patients on a general medical service.
Johns Hopkins Hosp Bull. 1966;119:299-315.
5.
Borda IT, Slone D, Jick H. Assessment of adverse reactions within a drug surveillance program.
JAMA. 1968;205:99-101.
6.
Miller RR. Hospital admissions due to adverse drug reactions: a report from the Boston
Collaborative Drug Surveillance Program. Arch
Intern Med. 1974;134:219-223. FULL
TEXT | ISI |
PUBMED
7.
Smith JW, Seidl LG, Cluff LE. Studies on the epidemiology of
adverse drug reactions, V: clinical factors influencing susceptibility. Ann
Intern Med. 1966;65:629-640. ISI |
PUBMED
8.
World Health Organization. International Drug Monitoring: The Role of the Hospital.
Geneva, Switzerland: World Health Organization; 1966. Technical
Report Series No. 425.
9.
Bates DB, Leape LL, Petrycki S. Incidence and preventability of
adverse drug events in hospitalized adults. J Gen
Intern Med. 1993;8:289-294. ISI |
PUBMED
10.
Karch FE, Lasagna L. Adverse drug reactions: a critical review.
JAMA. 1975;234:1236-1241. ABSTRACT
11.
Chalmers TC, Smith H, Blackburn B, et al. A method for
assessing the quality of a randomized
control trial. Control Clin Trials. 1981;2:31-49. FULL
TEXT | ISI |
PUBMED
12.
Karch FE, Smith CL, Kerzner B, Mazzullo JM, Weintraub M, Lasagna L. Adverse drug reactionsa matter of opinion. Clin Pharmacol Ther. 1976;19:489-92. ISI |
PUBMED
13.
Colditz GA, Burdick E, Mosteller F. Heterogeneity in meta-analysis of data from
epidemiologic studies: a commentary. Am J Epidemiol.
1995;142:371-382. FREE FULL
TEXT
14.
DerSimonian L, Laird N. Meta-analysis in clinical trials. Control
Clin Trials. 1986;7:177-188. FULL
TEXT | ISI |
PUBMED
15.
Berkey CS, Hoaglin DC, Mosteller F, Colditz GA. A
random-effects regression model for meta-analysis. Stat Med.
1995;14:395-411. ISI |
PUBMED
16.
Einarson TR. Drug-related hospital admissions.
Ann Pharmacother. 1993;27:832-840. ABSTRACT
17.
Rosner B. Fundamentals of Biostatistics. 4th ed. New York,
NY: Duxbury Press; 1995.
18.
Morgan K, Morgan S, Quitno N. Health Care State Rankings 1996. 4th ed. Lawrence, Kan:
Morgan Quitno Press; 1996.
19.
Bates DW, Boyle DL, Vander Vliet MB, Scheider J, Leape LL.
Relationship between medication errors and adverse drug reactions. J Gen Intern Med. 1995;10:199-205. ISI |
PUBMED
20.
Bates DW, Cullen DJ, Laird N, et al. Incidence of adverse drug events and potential
adverse drug events: implications for
prevention. JAMA. 1995;274:29-34. ABSTRACT
21.
Bowman L, Carlstedt BC, Black CD. Incidence of adverse drug reactions in adult medical inpatients. Can J Hosp Pharm.
1994;47:209-216. PUBMED
22.
Steel K, Gertman PM, Crescenzi C, Anderson J. Iatrogenic illness on
a general medical service at a university hospital. N Engl J
Med. 1981;304:638-642. ABSTRACT
23.
Mitchell AA, Goldman P, Shapiro S, Slone S. Drug utilization and reported
adverse reactions in hospitalized children. Am J
Epidemiol. 1979;110:196-204. FREE FULL TEXT
24.
Bennett BS, Lipman AG. Comparative study of prospective surveillance
and voluntary reporting in determining the incidence of adverse drug reactions. Am J Hosp Pharm.
1977;34:931-936. ABSTRACT
25.
May FE, Fuller S, Stewart RB. Drug use and adverse drug reactions prior to and
during hospitalization. J Am Pharm
Assoc. 1977;17:560-598. ISI |
PUBMED
26.
Miller RR. Drug surveillance utilizing epidemiological methods: a report
from the Boston Collaborative Drug Surveillance Program. Am J
Hosp Pharm. 1973;30:584-592. PUBMED
27.
McKenzie MW, Stewart RB, Weiss CF, Cluff LE. A pharmacist-based
study of the epidemiology of adverse drug reactions in pediatric medicine patients. Am J Hosp Pharm.
1973;30:898-903. PUBMED
28.
Wang R, Terry LC. Adverse drug reactions in a Veterans Administration hospital. J
Clin Pharmacol New Drugs. 1971;11:14-18. ISI |
PUBMED
29.
Gardner P, Watson LJ. Adverse drug reactions: a pharmacist-based
monitoring system. Clin Pharmacol Ther.
1970;11:802-807. ISI |
PUBMED
30.
Sidel VW, Koch-Weser J, Barnett GO, Eaton A. Drug utilization and adverse reactions in a general hospital.
Hospitals. 1967;41:80-88. PUBMED
31.
Reichel W. Complications in the care of five hundred elderly
hospitalized patients. J Am Geriatr Soc.
1965;13:973-977. ISI |
PUBMED
32.
Schimmel EM. The hazards of hospitalization. Ann Intern Med. 1964;60:100-110. ISI |
PUBMED
33.
Nelson KM, Talbert RL. Drug-related hospital admissions.
Pharmacotherapy. 1996;16:701-707. ISI |
PUBMED
34.
Col N, Fanale JE, Kronholm P. The role of medication noncompliance
and adverse drug reactions in hospitalizations of the elderly.
Arch Intern Med. 1990;150:841-845. ABSTRACT
35.
Mitchell AA, Lacouture PG, Sheehan JE, Kauffman RE, Shapiro S.
Adverse drug reactions in children leading to hospital admission.
Pediatrics. 1988;82:24-29. ISI |
PUBMED
36.
Bigby J, Dunn J, Goldman L, et al. Assessing the preventability of emergency
hospital admissions: a method for evaluating the quality of medical care
in a primary care facility. Am J
Med. 1987;83:1031-1036. FULL
TEXT | ISI |
PUBMED
37.
Lakshmanan MC, Hershey CO, Breslau D. Hospital admissions caused by
iatrogenic disease. Arch Intern Med. 1986;146:1931-1934. ABSTRACT
38.
Salem RB, Keane TM, Williams JG. Drug-related admissions to a Veterans'
Administration psychiatric unit.
Drug Intell Clin Pharm. 1984;18:74-76. ABSTRACT
39.
Stewart RB, Springer PK, Adams JE. Drug-related admissions to an
inpatient psychiatric unit. Am J
Psychiatry. 1980;137:1093-1095. ABSTRACT
40.
Frisk PA, Cooper JW, Campbell NA. Community-hospital pharmacist
detection of drug-related problems upon patient
admission to small hospitals. Am J Hosp Pharm.
1977;34:738-742. ABSTRACT
41.
McKenney JM, Harrison WL. Drug-related hospital admissions.
Am J Hosp Pharm. 1976;33:792-795. ABSTRACT
42.
McKenzie MW, Marchall GL, Netzloff ML, Cluff LE. Adverse drug reactions leading to hospitalization in children. J Pediatr.
1976;89:487-490. FULL
TEXT | ISI |
PUBMED
43.
Caranasos GJ, Stewart RB, Cluff LE. Drug-induced illness leading to hospitalization. JAMA.
1974;228:713-717. FULL
TEXT | ISI |
PUBMED
44.
Rawlins M, Thompson J. Mechanisms of
adverse drug reactions. In: Davies D, ed. Textbook of
Adverse Drug Reactions. 4th ed. Oxford,
England: Oxford University Press; 1991.
45.
Carbonin P, Pahor M, Bernabei R, Sgadari A.
Is age an independent risk factor of
adverse drug reactions in hospitalized medical patients? J Am Geriatr Soc.
1991;39:1093-1099. ISI |
PUBMED
46.
Hurwitz N, Wade OL. Intensive hospital
monitoring of adverse reactions to drugs. BMJ. 1969;1:531-536. PUBMED
47.
Ogilvie RI, Ruedy J. Adverse drug reactions during hospitalization. Can Med Assoc
J. 1967;97:1450-1457. PUBMED
48.
Domecq C, Naranjo CA, Ruiz I, Busto U. Sex-related variations
in the frequency and characteristics
of adverse drug reactions. Int J Clin Pharmacol Ther Toxicol.
1980;18:362-366. PUBMED
49.
Zilleruelo I, Espinoza E, Ruiz I. Influence of the assessment of the
severity on the frequency of adverse drug reactions. Int J Clin Pharmacol Ther Toxicol.
1987;25:328-333. PUBMED
50.
National Center for Health Statistics. National Hospital
Discharge Survey: Annual Summary, 1992. Hyattsville, Md: US Dept
of Health and Human Services; 1994. Publication 94-1779.
51.
Kahn L, Rubenstein L, Draper D, et al. The effects of
the DRG-based prospective payment system on quality of care for
hospitalized medicare patients. JAMA.
1990;264:1953-1955. ABSTRACT
52.
Hurwitz N. Predisposing factors in adverse drug reactions to drugs. BMJ. 1969;1:536-539. ISI |
PUBMED
53.
Smidt NA, McQueen EG. Adverse reactions to drugs: a comprehensive hospital
inpatient survey. N Z Med J.
1972;76:397-401. ISI |
PUBMED
54.
Smidt NA, McQueen EG. Adverse drug reactions in a general hospital. N Z Med
J. 1973;78:39. PUBMED
55.
O'Hara D, Carson N. Reporting of adverse events in hospitals in Victoria, 1994-1995. Med J
Aust. 1997;166:460-463. ISI |
PUBMED
56.
Evans RC, Classen DC, Horn SD, Bass SB, Burke JP.
Preventing adverse drug events in hospitalized patients. Ann Pharmacother.
1994;28:523-527. ABSTRACT
57.
Classen DC, Pestonik SL, Evans RS, Lloyd JF, Burke JP. Adverse drug events in hospitalized patients: excess length of stay, extra
costs, and attributable mortality. JAMA. 1997;277:301-306. ABSTRACT
58.
Bates DW, Spell N, Cullen DJ, et al. The costs of adverse drug events in hospitalized patients. JAMA.
1997;277:307-311. ABSTRACT
59.
American Hospital Association. Hospital Statistics,
1993-1994. Chicago, Ill: American Hospital Association; 1994.
RELATED
ARTICLES
This Week in
JAMA
JAMA. 1998;279:1145.
FULL
TEXT
Drugs and Adverse Drug Reactions: How Worried Should We
Be?
David W. Bates
JAMA.
1998;279:1216-1217.
EXTRACT
| FULL
TEXT
THIS ARTICLE HAS BEEN CITED BY OTHER
ARTICLES
 |
Escalating
polypharmacy
Gorard
QJM
2006;99:797-800.
ABSTRACT
| FULL
TEXT
Proceedings of the British Toxicology Society
Autumn Meeting University of York 20-22 September
1998
Hum Exp Toxicol
1999;18:46-65.
The FDA and Drug Safety: A Proposal for
Sweeping Changes
Furberg et
al.
Arch Intern Med
2006;166:1938-1942.
ABSTRACT
| FULL
TEXT
Frequency and Cost of Chemotherapy-Related Serious
Adverse Effects in a Population Sample of Women With
Breast Cancer
Hassett et al.
J Natl Cancer
Inst
2006;98:1108-1117.
ABSTRACT
| FULL
TEXT
Adverse Drug Reactions Among Children Over a
10-Year Period
Le et al.
Pediatrics
2006;118:555-562.
ABSTRACT
| FULL
TEXT
Adverse Drug Event Reporting in Intensive Care Units: A Survey of
Current Practices
Kane-Gill and Devlin
Ann Pharmacother
2006;40:1267-1273.
ABSTRACT
| FULL
TEXT
Drug Dosage Adjustments
According to Renal Function at Hospital
Discharge
van Dijk et al.
Ann Pharmacother
2006;40:1254-1260.
ABSTRACT
| FULL
TEXT
Genetic Testing in Cancer
Therapeutics
Ezzeldin and Diasio
Clin Cancer Res
2006;12:4137-4141.
FULL
TEXT
Metabolism of Efavirenz and 8-Hydroxyefavirenz by P450
2B6 Leads to Inactivation by Two Distinct Mechanisms
Bumpus et
al.
J. Pharmacol. Exp. Ther.
2006;318:345-351.
ABSTRACT
| FULL
TEXT
Enhanced Xenobiotic-Induced Hepatotoxicity and Kupffer
Cell Activation by Restraint-Induced Stress
Panuganti
et al.
J. Pharmacol. Exp. Ther.
2006;318:26-34.
ABSTRACT
| FULL
TEXT
Effect of internal reporting criteria on suspected
adverse drug reactions submitted to
MedWatch
Smith et al.
Am J Health Syst Pharm
2006;63:949-952.
FULL TEXT
Pharmacogenomics: from bedside to clinical practice
Marsh and
McLeod
Hum Mol Genet 2006;15:R89-R93.
ABSTRACT
| FULL
TEXT
The Development and Evaluation of an Integrated Electronic
Prescribing and Drug Management System for Primary
Care
Tamblyn et al.
J. Am. Med. Inform. Assoc.
2006;13:148-159.
ABSTRACT
| FULL
TEXT
Suboptimal Medication Use and Mortality in an Older Adult Community-Based
Cohort: Results From the Hispanic EPESE
Study
Espino et al.
J Gerontol A Biol
Sci Med Sci 2006;61:170-175.
ABSTRACT
| FULL
TEXT
Interethnic Differences in Genetic Polymorphisms of CYP2D6
in the U.S. Population:
Clinical
Implications
Bernard et al.
Oncologist
2006;11:126-135.
ABSTRACT
| FULL
TEXT
Adherence to Black Box Warnings for Prescription Medications
in Outpatients
Lasser et
al.
Arch Intern Med
2006;166:338-344.
ABSTRACT
| FULL
TEXT
Antibiotic Allergy
Gruchalla and
Pirmohamed
NEJM 2006;354:601-609.
FULL
TEXT
Adverse Drug Reactions in a Department of Pediatric
Surgery
Farrokhi et al.
J Trop Pediatr
2006;52:72-73.
FULL
TEXT
Improving the accuracy of patient
identification in the medication-use
process
Trapskin et al.
Am J Health Syst
Pharm 2006;63:218-222.
FULL TEXT
Where now for pharmacist led medication
review?
Holland et al.
J Epidemiol Community
Health 2006;60:92-93.
FULL
TEXT
Relationship of Medication Use to Health-Related Quality
of Life Among a Group of Older American Indians
Henderson et
al.
Journal of Applied Gerontology
2006;25:89S-104S.
ABSTRACT
Racializing Drug Design: Implications of
Pharmacogenomics for Health Disparities
Lee
Am J
Public Health 2005;95:2133-2138.
ABSTRACT
| FULL
TEXT
AUTOMATED ASSESSMENT OF TIME-DEPENDENT INHIBITION OF HUMAN CYTOCHROME P450
ENZYMES USING LIQUID CHROMATOGRAPHY-TANDEM MASS
SPECTROMETRY ANALYSIS
Atkinson et al.
Drug Metab. Dispos.
2005;33:1637-1647.
ABSTRACT
| FULL
TEXT
Adverse Drug Reactions in Hospitalized Children in a Department of Infectious
Diseases
Fattahi et al.
J Clin Pharmacol
2005;45:1313-1318.
FULL
TEXT
Identification of Inappropriate Drug Prescribing by Computerized, Retrospective DUR
Screening in Korea
Yeom et
al.
Ann Pharmacother 2005;39:1918-1923.
ABSTRACT
| FULL
TEXT
Drug-Related Visits to the Emergency
Department
Zed
Journal of Pharmacy Practice
2005;18:329-335.
ABSTRACT
Pharmacogenetics/genomics and personalized
medicine
Sadee and
Dai
Hum Mol Genet 2005;14:R207-R214.
ABSTRACT
| FULL
TEXT
Adverse Drug Reactions and Avalanches: Life at the
Edge of Chaos
Frattarelli
J Clin Pharmacol
2005;45:866-871.
ABSTRACT
| FULL
TEXT
The Efficacy and Safety of Pain Management Before and After
Implementation of Hospital-Wide Pain Management Standards: Is Patient
Safety Compromised by Treatment Based Solely on Numerical
Pain Ratings?
Vila et
al.
Anesth Analg 2005;101:474-480.
ABSTRACT
| FULL
TEXT
Pharmacogenomics: Harbinger for the Era of Personalized
Medicine?
Sadee
Mol
Interv
2005;5:140-143.
FULL
TEXT
Epidemiology, Comparative Methods of Detection, and
Preventability of Adverse Drug Events
Al-Tajir and
Kelly
Ann Pharmacother 2005;39:1169-1174.
ABSTRACT
| FULL
TEXT
Accuracy of adverse-drug-event reports collected
using an automated dispensing system
Romero and
Malone
Am J Health Syst Pharm
2005;62:1375-1380.
ABSTRACT
| FULL
TEXT
Cardiovascular Pharmacogenomics: Current Status, Future
Prospects
Anderson et al.
J CARDIOVASC PHARMACOL
THER 2003;8:71-83.
ABSTRACT
Challenging times for food allergy
tests
Roberts
Arch. Dis. Child.
2005;90:564-566.
FULL
TEXT
Drug Metabolism and Variability among
Patients in Drug
Response
Wilkinson
NEJM
2005;352:2211-2221.
FULL
TEXT
Five Years After To Err Is Human: What Have We
Learned?
Leape and Berwick
JAMA
2005;293:2384-2390.
ABSTRACT
| FULL
TEXT
The Research on Adverse Drug Events and Reports (RADAR)
Project
Bennett et al.
JAMA
2005;293:2131-2140.
ABSTRACT
| FULL
TEXT
Concealed Renal Insufficiency and Adverse Drug Reactions in Elderly Hospitalized Patients
Corsonello et
al.
Arch Intern Med
2005;165:790-795.
ABSTRACT
| FULL
TEXT
Emergency Physician Recognition of Adverse Drug-related Events in Elder Patients Presenting to an Emergency
Department
Hohl et al.
Acad Emerg Med
2005;12:197-205.
ABSTRACT
| FULL
TEXT
Pharmacogenetics, ethical issues: review of the Nuffield
Council on Bioethics Report
Corrigan
J Med
Ethics 2005;31:144-148.
ABSTRACT
| FULL
TEXT
Service utilization in 1896 and 1996: morbidity and
mortality data from North Wales
Healy et
al.
History of Psychiatry 2005;16:27-41.
ABSTRACT
Safety Issues in the Interaction of Conventional,
Complementary, and Alternative Health Care
Curtis and
Gaylord
Complementary Health Practice Review
2005;10:3-31.
ABSTRACT
Unresolved issues in
pharmacy
Zellmer
Am J Health Syst Pharm
2005;62:259-265.
FULL TEXT
Healthy ageing: ageing
safely
Buckley
EHJ Suppl
2001;3:N6-N10.
ABSTRACT
Codeine Intoxication Associated with
Ultrarapid CYP2D6 Metabolism
Gasche et
al.
NEJM 2004;351:2827-2831.
ABSTRACT
| FULL
TEXT
Non-compliance: a side effect of drug information leaflets
Verdu
and Castello
J Med Ethics 2004;30:608-609.
FULL
TEXT
Potential for Conflict of Interest in the Evaluation of Suspected
Adverse Drug Reactions: A Counterpoint
Strom
JAMA
2004;292:2643-2646.
FULL
TEXT
Moving Toward Integrative Care: Rationales, Models,
and Steps for Conventional-Care Providers
Mann et
al.
Complementary Health Practice Review
2004;9:155-172.
ABSTRACT
The Tolling of the Bell: Women's Health,
Women's Rights
Dickerson
Obstet Gynecol
2004;104:653-657.
FULL
TEXT
Patient reports of adverse events associated with
acupuncture treatment: a prospective national
survey
MacPherson et al.
Qual Saf Health
Care 2004;13:349-355.
ABSTRACT
| FULL
TEXT
Antidepressants: An Avoidable and Solvable
Controversy
Cohen
Ann Pharmacother
2004;38:1743-1746.
FULL
TEXT
A Drug Database Model as a Central
Element for Computer-Supported Dose Adjustment within a CPOE
System
Martin et al.
J. Am. Med.
Inform. Assoc.
2004;11:427-432.
ABSTRACT
| FULL
TEXT
Pharmacogenomics and Pharmacogenetics: Future Role of
Molecular Diagnostics in the Clinical Diagnostic
Laboratory
Ito and Demers
Clin Chem
2004;50:1526-1527.
FULL
TEXT
Service innovations: Pharmacogenetic
clinics in psychiatry: a clinical reality?
Hodgson et
al.
Psychiatr Bull 2004;28:298-300.
ABSTRACT
| FULL
TEXT
Open letter to Annette King, Minister of Health, New
Zealand
Herxheimer
BMJ
2004;329:51-51.
FULL TEXT
Adverse drug reactions as cause of admission to
hospital: prospective analysis of 18 820 patients
Pirmohamed et
al.
BMJ 2004;329:15-19.
ABSTRACT
| FULL
TEXT
Direct reporting of suspected adverse drug reactions by patients
Choonara
J
R Soc Med 2004;97:316-317.
FULL TEXT
Policy, the Public, and Priorities in Alternative Medicine
Research
JONAS
The ANNALS of the American Academy
of Political and Social Science 2002;583:29-43.
ABSTRACT
Using a Computerized Drug Prescription Screening System to Trace Drug Interactions in an Outpatient Setting
Morera et
al.
Ann Pharmacother 2004;38:1301-1306.
ABSTRACT
| FULL
TEXT
Using Pharmacogenetics to Improve
Drug Safety and
Efficacy
Haga and Burke
JAMA
2004;291:2869-2871.
FULL
TEXT
Terminology and the Construction of
Scientific Disciplines: The Case of
Pharmacogenomics
Hedgecoe
Science Technology
Human Values 2003;28:513-537.
ABSTRACT
Herbal Remedies in the United States: Potential
Adverse Interactions With Anticancer
Agents
Sparreboom et al.
J Clin Oncol
2004;22:2489-2503.
ABSTRACT
| FULL
TEXT
Policy Implications of Research on Nurse
Staffing and Quality of Patient
Care
Buerhaus and Needleman
Policy Politics
Nursing Practice
2000;1:5-15.
ABSTRACT
Aging Biology and Geriatric
Clinical Pharmacology
McLean
and Le Couteur
Pharmacological Reviews
2004;56:163-184.
ABSTRACT
| FULL
TEXT
Medications as Social Phenomena
Cohen et
al.
Health (London) 2001;5:441-469.
ABSTRACT
VALIDATED ASSAYS FOR HUMAN CYTOCHROME P450
ACTIVITIES
Walsky and Obach
Drug Metab. Dispos.
2004;32:647-660.
ABSTRACT
| FULL
TEXT
Evidence and the Future of Medicine
Mike
Eval
Health Prof 2003;26:127-152.
ABSTRACT
Comparison of Drug-Related Problems in Different Patient
Groups
Viktil et al.
Ann Pharmacother
2004;38:942-948.
ABSTRACT
| FULL
TEXT
Rapid Identification of Dihydropyrimidine Dehydrogenase Deficiency by
Using a Novel 2-13C-Uracil Breath
Test
Mattison et al.
Clin Cancer Res
2004;10:2652-2658.
ABSTRACT
| FULL
TEXT
Patient Safety Forum: Developing a Strategic Plan: What is a
reasonable first-year strategic plan to better address medication
errors in psychiatry?
Conway et
al.
PS 2004;55:259-263.
ABSTRACT
| FULL
TEXT
Adverse Events Due to
Discontinuations in Drug Use and Dose Changes
in Patients Transferred Between Acute and
Long-term Care Facilities
Boockvar et al.
Arch
Intern Med
2004;164:545-550.
ABSTRACT
| FULL
TEXT
Pharmacoeconomic analysis of thiopurine methyltransferase polymorphism
screening by polymerase chain reaction for treatment with
azathioprine in Korea
Oh et
al.
Rheumatology 2004;43:156-163.
ABSTRACT
| FULL
TEXT
Why Aren't Lower, Effective, OTC Doses Available Earlier
by Prescription?
Cohen
Ann Pharmacother
2003;37:136-142.
ABSTRACT
| FULL
TEXT
Pediatric Drug Research -- The Road Less
Traveled
Holdsworth
Ann Pharmacother
2003;37:586-591.
FULL
TEXT
Drug-Induced Esophageal Injuries and
Dysphagia
O'Neill and Remington
Ann Pharmacother
2003;37:1675-1684.
ABSTRACT
| FULL
TEXT
Adverse Drug Events Associated with Hospital
Admission
Peyriere et al.
Ann Pharmacother
2003;37:5-11.
ABSTRACT
| FULL
TEXT
Updating the Beers Criteria for Potentially
Inappropriate Medication Use
in Older Adults: Results of a US
Consensus Panel of Experts
Fick et al.
Arch
Intern Med
2003;163:2716-2724.
ABSTRACT
| FULL
TEXT
The Impact of Dedicated Medication Nurses on the
Medication Administration Error Rate: A Randomized
Controlled Trial
Greengold et al.
Arch
Intern Med
2003;163:2359-2367.
ABSTRACT
| FULL
TEXT
Delayed Drug Hypersensitivity Reactions
Pichler
Ann
Intern Med
2003;139:683-693.
ABSTRACT
| FULL
TEXT
Inflammation and Drug Idiosyncrasy--Is There a
Connection?
Roth et al.
J. Pharmacol. Exp.
Ther. 2003;307:1-8.
ABSTRACT
| FULL
TEXT
Pharmacists on Rounding Teams Reduce Preventable
Adverse Drug Events in Hospital General Medicine Units
Kucukarslan et
al.
Arch Intern Med
2003;163:2014-2018.
ABSTRACT
| FULL
TEXT
The medical office of the 21st century (MOXXI):
effectiveness of computerized decision-making support in reducing inappropriate prescribing in primary care
Tamblyn et
al.
Can Med Assoc J 2003;169:549-556.
ABSTRACT
| FULL
TEXT
Denaturing High Performance Liquid
Chromatography Analysis of the DPYD Gene in Patients with Lethal 5-Fluorouracil
Toxicity
Ezzeldin et al.
Clin Cancer Res
2003;9:3021-3028.
ABSTRACT
| FULL
TEXT
Quality Assessment of a Collaborative Approach for
Decreasing Drug-Related Morbidity and
Achieving Therapeutic
Goals
Isetts et al.
Arch Intern Med
2003;163:1813-1820.
ABSTRACT
| FULL
TEXT
Drug-Induced
Hepatotoxicity
Lee
NEJM
2003;349:474-485.
FULL
TEXT
Failures of the therapeutic chain as a cause of drug ineffectiveness
Figueras
and Laporte
BMJ 2003;326:895-896.
FULL TEXT
Adverse Drug Events in Ambulatory Care
Gandhi
et al.
NEJM 2003;348:1556-1564.
ABSTRACT
| FULL
TEXT
Drug-Drug Interactions Among Elderly
Patients Hospitalized for Drug
Toxicity
Juurlink et al.
JAMA
2003;289:1652-1658.
ABSTRACT
| FULL
TEXT
Prioritizing Strategies for
Preventing Medication Errors and
Adverse Drug Events in Pediatric Inpatients
Fortescue et
al.
Pediatrics 2003;111:722-729.
ABSTRACT
| FULL
TEXT
The Safety of Newly Approved Medicines: Do Recent Market Removals Mean
There Is a Problem?
Friedman et al.
JAMA
1999;281:1728-1734.
ABSTRACT
| FULL
TEXT
Avoiding the Unintended Consequences of Growth
in Medical Care: How Might More Be
Worse?
Fisher and Welch
JAMA
1999;281:446-453.
ABSTRACT
| FULL
TEXT
Time for Action on Drug Safety
Burkhart et
al.
JAMA 1999;281:319-322.
FULL
TEXT
Adverse Drug Reactions in Hospitalized Patients
Fremont-Smith et
al.
JAMA 1998;280:1741-1741.
FULL
TEXT
A Computer Alert System to Prevent Injury From Adverse Drug Events: Development and
Evaluation in a Community Teaching Hospital
Raschke et
al.
JAMA 1998;280:1317-1320.
ABSTRACT
| FULL
TEXT
Association Between Licensing Examination Scores and Resource Use and
Quality of Care in Primary Care
Practice
Tamblyn et al.
JAMA
1998;280:989-996.
ABSTRACT
| FULL
TEXT
Time to Act on Drug Safety
Moore et
al.
JAMA 1998;279:1571-1573.
FULL
TEXT
Drugs and Adverse Drug Reactions: How Worried Should We
Be?
Bates
JAMA
1998;279:1216-1217.
FULL
TEXT
Depression and Adverse Drug Reactions Among Hospitalized Older
Adults
Onder et al.
Arch Intern Med
2003;163:301-305.
ABSTRACT
| FULL
TEXT
Incidence and Impact of Adverse Drug Events in Pediatric Inpatients
Holdsworth et
al.
Arch Pediatr Adolesc Med 2003;157:60-65.
ABSTRACT
| FULL
TEXT
|

